Robitussin Recall 2024 Formulary – Haleon is voluntarily recalling eight lots of Robitussin Honey CF Max Day Adult and Robitussin Honey CF Max Nighttime Adult due to potential microbial contamination. . A Robitussin recall is underway for two of the brand’s products after discovering microbial contamination of the cough syrups. Haleon (NYSE:HLN), the maker of Robitussin, notes that the recall affects .
Robitussin Recall 2024 Formulary
Source : patents.google.com
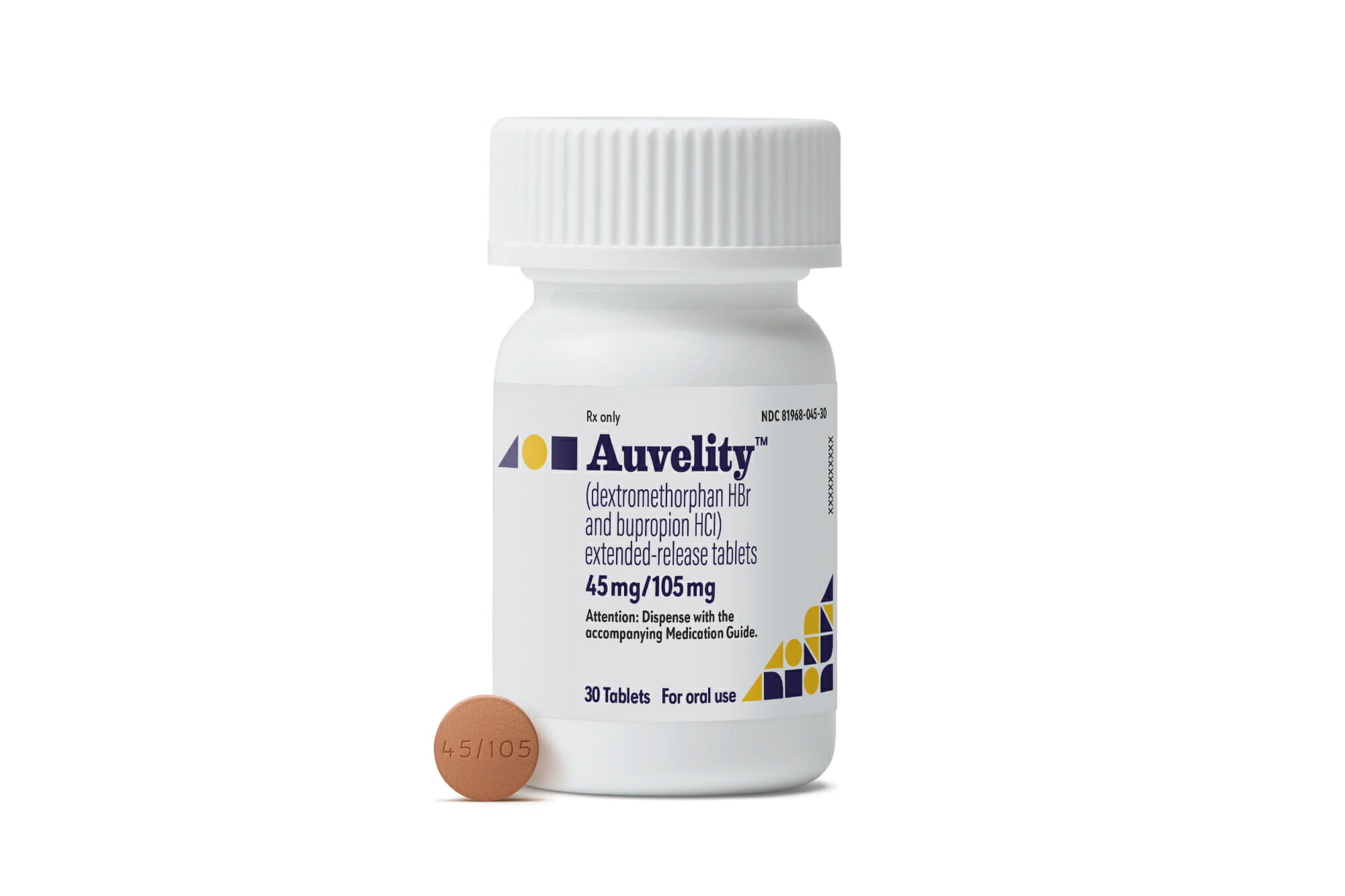
FDA Approves Rapid Acting Auvelity for Major Depression
Source : www.formularywatch.com
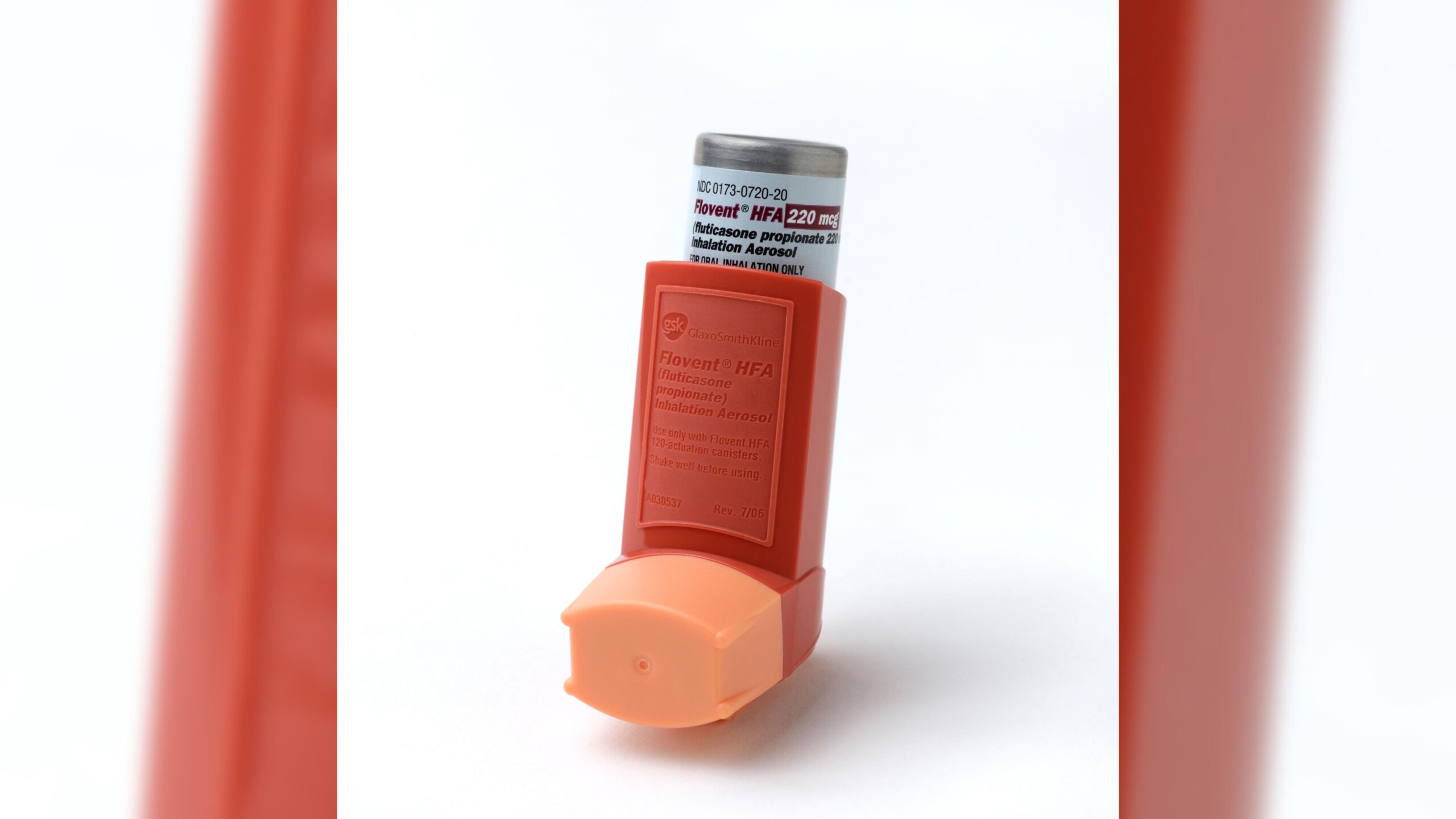
A huge shock to the system’: Doctors warn about asthma inhaler
Source : ksltv.com
AIS Healthcare Celebrates National Pharmacist Day
Source : finance.yahoo.com
A huge shock to the system’: Doctors warn about asthma inhaler
Source : kslnewsradio.com
American Society for Clinical Pharmacology and Therapeutics 2023
Source : ascpt.onlinelibrary.wiley.com
Amazon.com: Medique Dover 2125323 Sudanyl PE Decongestant Tablets
Source : www.amazon.com
Amazon.com: Andi Lynn’s Wild Cherry Bark Syrup Bronchial Wellness
Source : www.amazon.com
Amazon.com: Elderberry Cough Syrup
Source : www.amazon.com
WO2020178653A1 Esketamine for the treatment of depression
Source : patents.google.com
Robitussin Recall 2024 Formulary WO2020178653A1 Esketamine for the treatment of depression : WASHINGTON (AP) — The maker of Robitussin cough syrup is recalling several lots of products containing honey due to contamination that could pose a serious risk to people with weakened immune systems. . Eight lots of two kinds of Robitussin cough syrups have been recalled for “a microbial contamination.” That’s from the FDA-posted recall notice by manufacturer Haleon, which doesn’t describe how the .